With over 30 depots in the Americas, EMEA, and APAC regions, we can store and efficiently distribute your drug candidate to any location across the world, including countries that are difficult to reach.
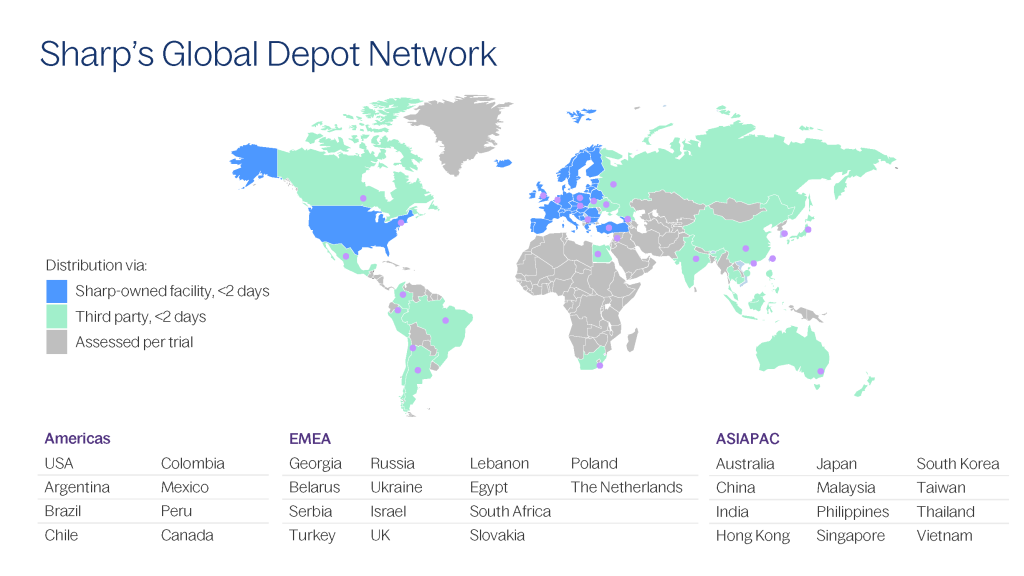
Our experts understand the complexities of clinical trial storage and distribution and are committed to quality and on-time in-full delivery. Through our consultative approach, we offer customs and regulatory guidance on moving clinical trial material compliantly around the globe.
We have robust cold-chain capabilities, offering ambient, chilled, and ultra-low frozen storage and distribution, ensured by validated temperature monitoring devices.
From multinational clinical trials to direct-to-patient logistics, we can meet your storage and distribution needs.
Solutions
- Pre-qualified temperature-controlled packaging
- Controlled ambient storage and distribution between 15-25°C
- Chilled storage and distribution between 2-8°C
- Frozen storage and distribution to -80°C
- Ultra-low frozen storage and distribution
- Validated temperature monitoring devices
- Optimized shipment route selection
- Courier selection and management
- Barcode quality control systems
- Importer of Record (IoR) services where regulations allow
- Client portal for inventory and shipment monitoring
- Clinventory inventory management system (IMS)
- Clinteract interactive response technology (IRT)


