About this webinar:
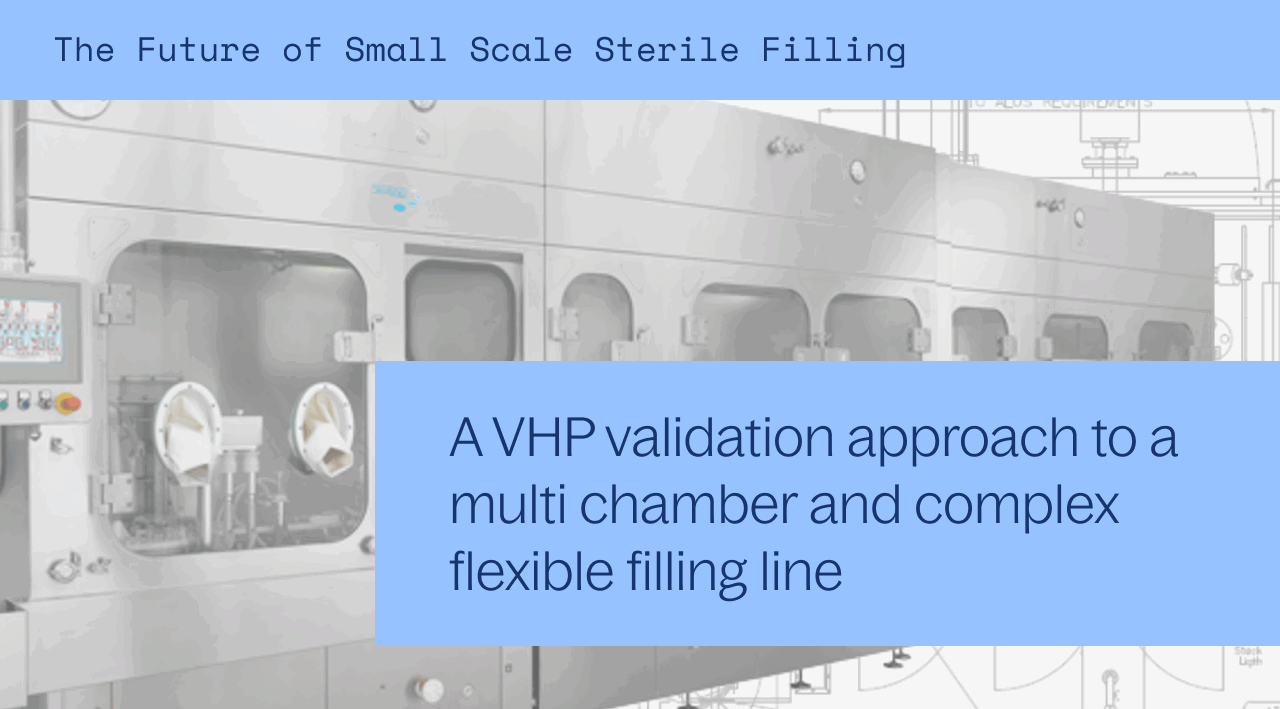
Isolator technology is now the expectation in fill finish. The sterility assurance this technology offers greatly reduces contamination risk and offers clients a much higher quality product.
But, how do you validate a multi-chamber filling line with multiple pieces of complex equipment? How do you ensure that a sufficient sanitization level is met?
In this one hour webinar, Sharp Sterile Manufacturing (formerly Berkshire Sterile Manufacturing) and Steriline will discuss their validation approach for SSM’s new filling line that will begin operations in 2022. This new line will be validated to a 6-log reduction in geobacillus spores, which is far greater than any SAL that could be reached with a closed RABs system.